EML4-ALK and KIF5B-ALK Fusion Gene Detection Test
A Comprehensive, Rapid and Reliable ALK Real-Time PCR Assay
Research Use Product
Research Service
Early Detection/Screening
Diagnosis
Therapy Selection
Therapy Monitoring
Introducing QFusion™ Test
QFusion™ ALK Fusion Gene Detection Kits provide an easy-to-use multiplex RT-qPCR assay that simultaneously detects the most prevalent EML4-ALK and KIF5B gene fusion breakpoints. This highly specific and sensitive assay requires a minimal amount of RNA, and it is a rapid and reliable alternative to the laborious FISH test.
Cover 95% EML4-ALK and 90% KIF5B-ALK variants in COSMIC database
One-tube multiplex qPCR reactions and only 50 ng RNA as assay input
No cross-reactivity with 350 ug WT RNA and successful detection with 50 copies of fusion templates
Fast turnaround time: total turnaround time approximately 2.5 hours
EML4-ALK has been emerged as one of the most important driver oncogenes in lung cancer and the first targeted fusion
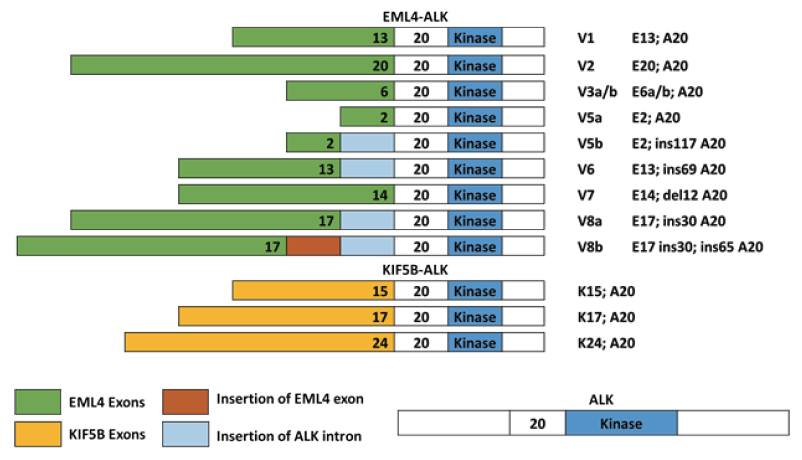
ALK Fusion Gene Variants Detected
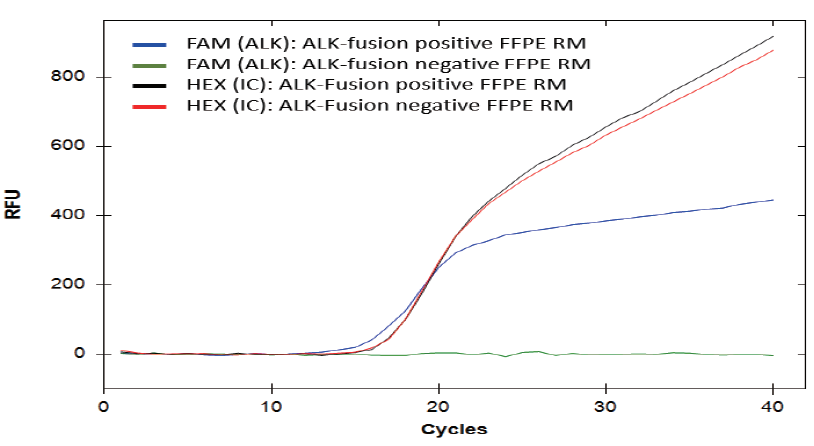
Amplification Curve Generated by QFusion™ for FFPE Reference Materials
EML4-ALK fusion gene variant 1 could be successfully detected from the ALK fusion-positive FFPE reference material (RM) (blue line) but not the negative RM (green line). The internal control (IC) assay similarly amplified the positive and negative FFPE RMs (black and red lines), indicating that the total RNA input (50 ng) extracted from these RMs was about the same.
Product Specifications for QFusion™ EML4-ALK and KIF5B-ALK Fusion Gene Detection Test
Product Catalog Number
CE/IVD Catalog Number: DC-20-0020E;
Research-Use-Only (RUO) Catalog Number: DC-20-0020R
Intended Use
For in vitro diagnostic use (CE/IVD) or for research use
Sample Type
FFPE tissues from non-small cell lung cancer (minimum 50 ng RNA)
Fusion Variants
EML4-ALK: V1, V2, V3a/b, V5a/b, V6,V7, V8a/b KIF5B-ALK: (K15; A20), (K17; A20), (K24; A20)
Pack Size
20 Samples
Instruments Validated
Roche LightCycler® 96, Roche LightCycler® 480 II, and Bio-Rad CFX384
Detection Channel
FAM; HEX
Turnaround Time
PCR setup: ~0.5 hour; assay run time: ~1 hour
Stability
Stable for 12 Months at -25°C to -15°C
