Anti-SARS-CoV-2 IgG Test Kit
An immunoassay test intended for the detection of anti-SARS-CoV-2 IgG from serum or plasma from individuals.
Research Use Product
The QuantiVirus™ Anti-SARS-CoV-2 IgG Test is an immunoassay test intended for the detection of anti-SARS-CoV-2 IgG from serum or plasma from individuals suspected of COVID-19 by their healthcare provider. This test is to check if the COVID-19 virus antibody is present due to virus infection.
Product Highlights
- Outside of the US: CE Marked
- US: For Research Use Only. Not for use in diagnostic procedures
- High throughput: 78 samples per run
- Sample type: Serum or EDTA plasma
- Sample input: 10 uL
- Turnaround time: 3 hours
Validated Machines
Luminex 200 and MAGPIX
COVID-19 Hotline
Email: support@diacarta.com
Phone #: +1 510-878-6662, option 4 (tech support)
GET A QUOTE NOW
Product Configuration
Kit Components
- Capture beads
- 20x anti-human IgG antibody
- Positive control and negative control
- Wash buffer and assay buffer
- 96-well plate and plate seals
Pack Size
96-reaction per kit, 384-reaction per kit, 960-reaction per kit

*This product configuration image only shows the 96-reaction pack size. Please refer to product IFU for 384-reaction and 960-reaction pack size kit configuration.
How Does the QuantiVirus™ Anti-SARS-CoV-2 IgG Test Kit Work?
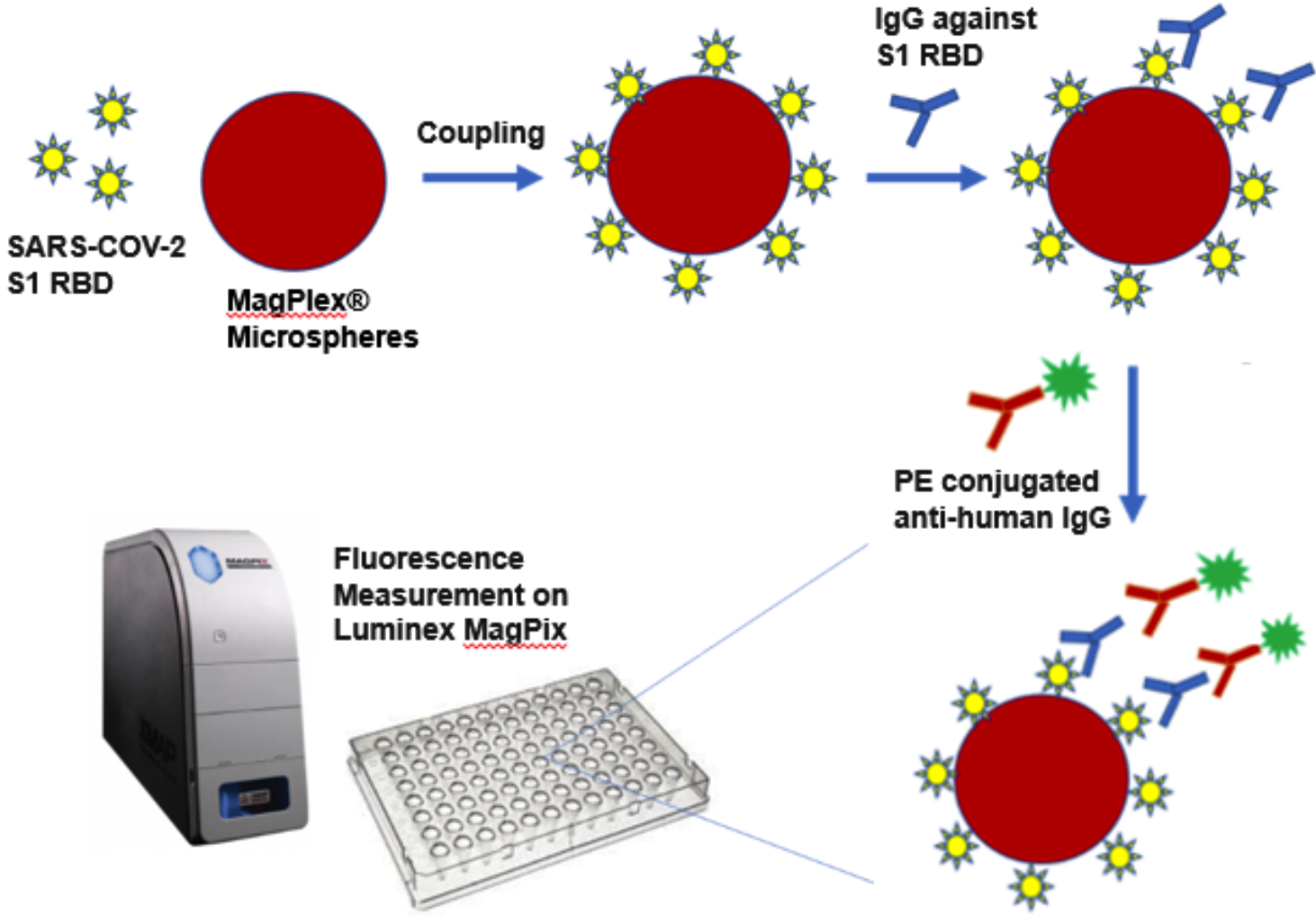
First, S1 RBD coated beads and human specimens are mixed and incubated at room temperature for 1 hour, allowing the coated beads to capture the IgG antibodies present in human specimens.
After washing, Phycoerythrin (PE) conjugated anti-human IgG is added to reaction mixture and incubated at room temperature for 30 minutes.
After washing, PE fluorescence of each well in a 96-well plate is measured on Luminex MagPix® platform as Median Fluorescence Intensity (MFI).

Step 1
Capture beads and sample incubation at room temperature (1 hour)

Step 2
Anti-human IgG antibody incubation at room temperature (30 minutes)
Step 3
Plate reading & data acquisition
Ordering Information
Customer Ordering Information
Product Name: QuantiVirus™ Anti-SARS-CoV-2 IgG Test
96-Reaction Kit Catalog Number (CE Marked): DC-11-0022E
384-Reaction Kit Catalog Number(CE Marked): DC-11-0023E
960-Reaction Kit Catalog Number(CE Marked): DC-11-0024E
*This device/product is not EUA authorized and it is available in the United States for Research Use Only (RUO). Not for use in diagnostic procedures.
COVID-19 Total Solution
DiaCarta offers a COVID-19 total solution to support the fight against COVID-19, including the RT-PCR test kit, antibody IgG test kit, and CLIA lab service.

QuantiVirus™ SARS-CoV-2 Test Kit (RT-PCR Test - Detects 3 genes)

