QuantiDNA™ cfDNA Quantification
Direct cfDNA Test for Research and Clinical Studies
DiaCarta’s Direct cfDNA Quantification Tests, available as QuantiDNA™ assays, measure the ng/ml level of cfDNA concentration in human plasma using a luminometer (QuantiDNA™ DNA Measurement Assay) and Luminex MAGPIX (QuantiDNA™ Direct cfDNA Test). Unlike the majority of the cfDNA quantification methods that need to extract DNA first before measuring the DNA concentration, our Direct cfDNA Quantification Tests do not need the isolation of cfDNA for quantitation and provide more reliable results because it eliminates DNA loss during DNA extraction. In addition, it uses only a few microliters of plasma for cfDNA quantitation.
QuantiDNA™ could also be used as a liquid biopsy triage tool for pan-cancer radiation or chemotherapy.
QuantiDNA™ DNA Measurement Assay
Signal detection by a luminometer
Pack Size: 96 Reactions
Research Service is also available.
QuantiDNA™ Direct cfDNA Test
Signal detection by Luminex MAGPIX
Pack Size: 96 Reactions
Research Service is also available.
Input DNA
10 µl plasma
GET A QUOTE NOW
Personalized Medicine and Precision Medicine
The future trend for healthcare is personalized medicine (tailoring of medical treatment based on
Liquid Biopsy as a Tool for Precision Medicine
As a personalized medicine tool,
Rapid growth in liquid biopsy analysis, especially analysis of circulating cell-free DNA (cfDNA) provides valuable information in disease diagnosis and therapeutics: early diagnosis, risk assessment for metastatic relapse or metastatic progression, patients stratification, disease real-time monitoring during therapies, identification of somatic mutations for different therapeutic targets, drug sensitivity and resistance understanding, and disease progression such as metastasis development, and many others.
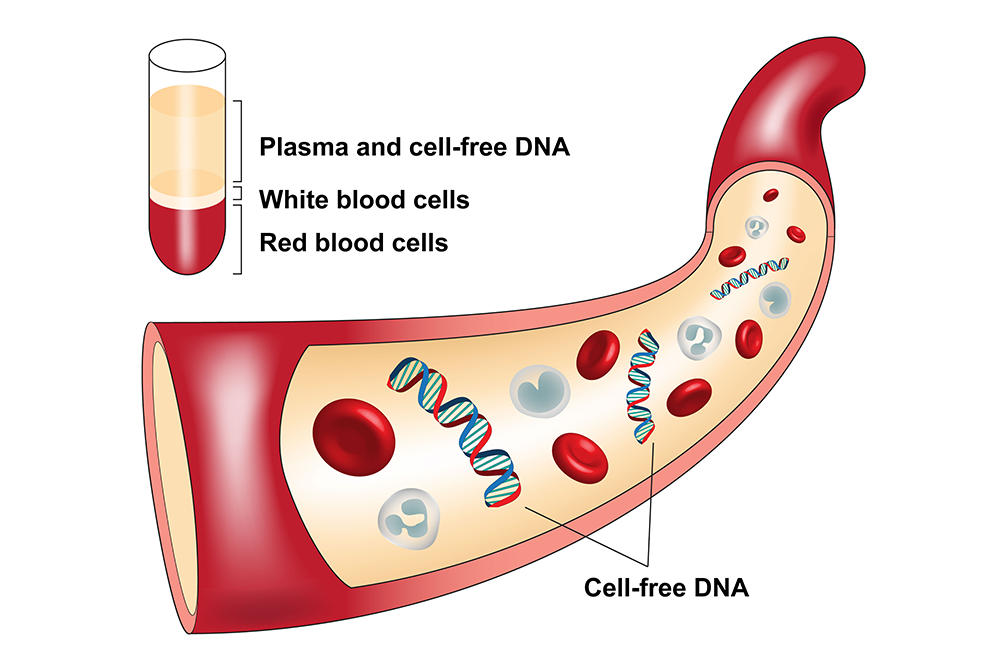
What is cfDNA?
cfDNA is commonly found circulating in plasma. First discovered in plasma in 1948 by Mandel and
It is thought that cfDNA comes from apoptosis (programmed cell death) and cell necrosis. The size of the cfDNA varies depending on the source. It is reported that cfDNA from apoptosis can be 100 bp to 500 bp, average about 170 to 180 bp while the cfDNA from necrosis is usually much bigger and can reach more than 1 kb.
What is the Correlation between the Levels of cfDNA and Diseases?
The publications for cfDNA from plasma have been increasing over the years from <100 in 2007 to >300 in 2016. cfDNA has been studied as a potential biomarker for different diseases including, but not limited to cancer, cardiovascular diseases (sepsis, myocardial infarction), hemodialysis, inflammation, infection, ischemic stroke, and connective tissue diseases, pregnancy-associated disorders and transplantation rejection.
Tumors shed DNA in blood streams, called circulating tumor DNA (ctDNA). Although ctDNA is only 0.01 to 0.1% of the total cfDNA, presence of the ctDNA in blood provides good liquid biopsy samples for testing of genetic mutations and epigentic changes. Studies show that the amount of cfDNA and the ctDNA in plasma is proportionally correlated and cfDNA quantitation can be used as an indicator for ctDNA quantitation because ctDNA level is really low in plasma and accurate quantification is difficult. Research indicates that the level of cfDNA in cancer subjects is higher than that in the healthy individuals. The later stages of the cancer subjects have much higher level of cfDNA than those with early stages of the same cancer. Read our white paper for review for more details.
Quantitative cfDNA Analysis: Lack of Standardization
cfDNA quantification for various diseases including cancer, cardiovascular disseases, infection and autoimmune diseases and their treatment indicate that the cfDNA levels may be used as a potential biomarker and have been shown to be a good biomarker, for instance, for tumor burden. However, lack of standardization in cfDNA quantitation makes comparison of different studies difficult. In most of the studies, cfDNA is extracted and purified first, followed by assessment. This adds variation to the cfDNA quantitation. Although cfDNA is a potential biomarker for disease prediction, diagnosis and prognosis, only limited studies have firm conclusions. Standardization of cfDNA quantitation method will be critical for future correlation studies of cfDNA quantitation with different diseases and demonstration of cfDNA used as a potential disease biomarker.
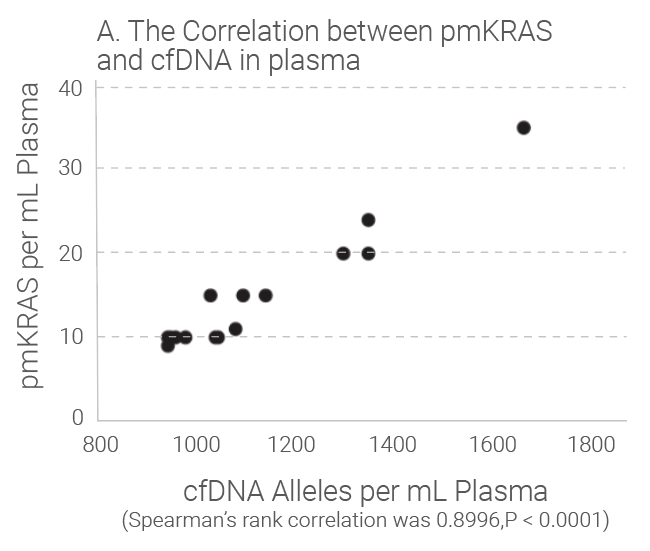
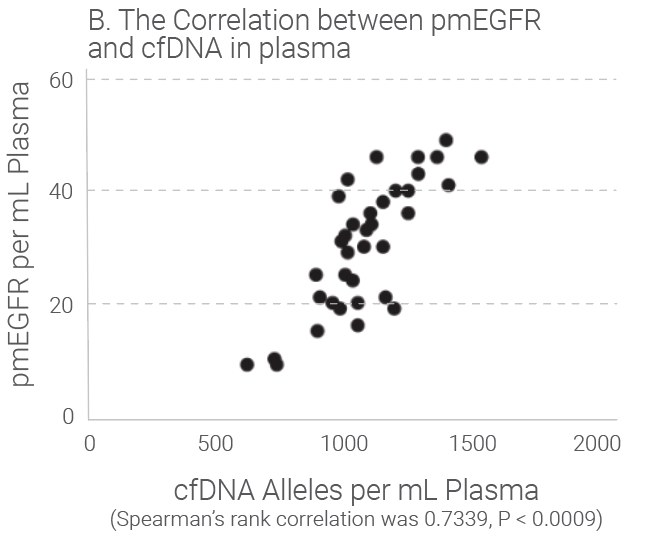
Quantitation Methods of cfDNA
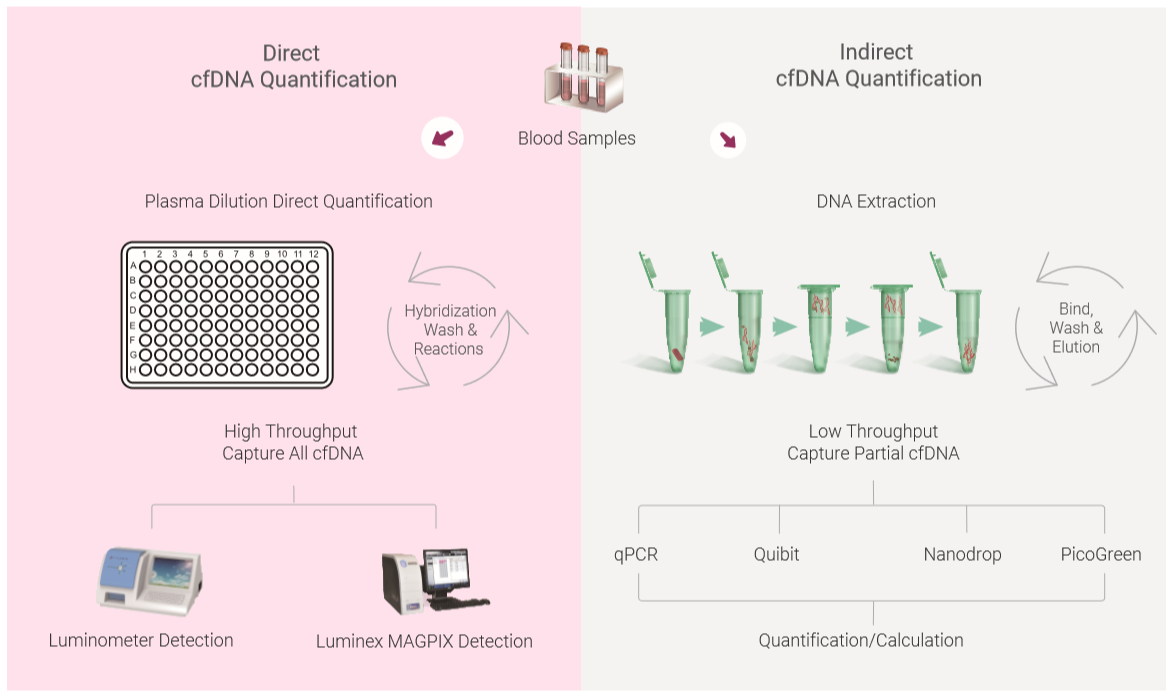
Although many publications quantify cfDNA for different diseases studies, the lack of a standard for quantitation of cfDNA has generated different cfDNA results, especially for the methods that are involved different DNA extraction methods and following assessment and calculation. The cfDNA quantitation method can be classified as direct quantitation and indirect quantitation methods. Direct quantitation of cfDNA directly measures cfDNA concentration without the need for DNA extraction/purification, such as direct PCR, direct SYBR Gold assay, or
Compared to indirect quantification, the advantage of the using direct quantitation methods include:
The cfDNA extraction and purification is not necessary for determination of the concentration
cfDNA concentration measurement needs minimum of plasma rather large amount of plasma
Reagents used in DNA extraction may affects the quantitation results in the following procedures
The procedure minimizes loss of cfDNA during cfDNA purification
The procedure saves hands-on time and may get adapted to automation easily for high throughput needs
Below figure shows the comparison of direct and indirect DNA quantification methods.
Suggested Workflow for cfDNA Quantification for Real-Time cfDNA Level Monitoring
As we reviewed above, cfDNA has been widely studied for
Comparison of healthy individuals and various types of patients
Disease stage and treatment monitoring for a certain type of patients
If the cfDNA does not need to be purified for further mutation or epigenetic change detection,
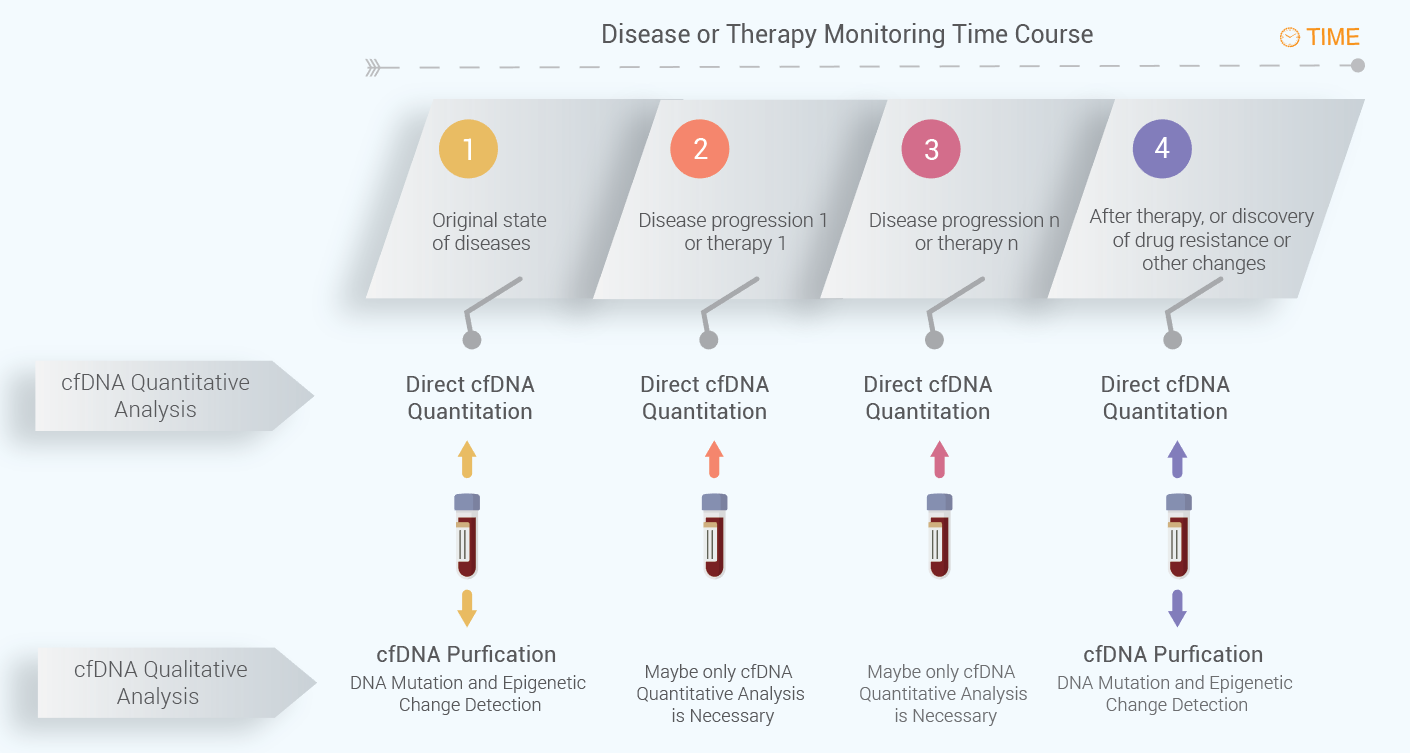
The workflow for cfDNA quantitation is summarized below. At different time of points at disease progression or therapy, cfDNA can be quantitated using the direct cfDNA quantitation kits. However, it is not always necessary to extract cfDNA for the quantitation purpose if no cfDNA changes need to be detected at different time points. Often, the cfDNA qualitative analysis is necessary for diagnosis of diseases for particular genetic changes or therapy guidance based on certain gene mutations, such as drug resistance development after use of first or second generation of tyrosine kinase inhibitors (TKIs). Below figure shows the suggested workflow for cfDNA monitoring for disease progression or therapy.
Instrument Required for QuantiDNA™ cfDNA Test
QuantiDNA™ DNA Measurement Assay (Luminometer Platform)
Plate Incubator
Requirement: Capable of maintaining a constant temperature between 46–55 °C ±1 °C)
Validated Machine: Thermo Shaker Incubator
Luminometer
Requirement: read 420nm wavelength, read time of 0.2 seconds/well, linear dynamic range ≥ 5-log (optional) and well-to-well uniformity < ± 5%)
Validated Machine: DiaCarta Luminometer
QuantiDNA™ Direct cfDNA Test (Luminexr Platform)
Plate Incubator
Requirement: Capable of maintaining a constant temperature between 46–55 °C ±1 °C)
Validated Machine: Thermo Shaker Incubator
Luminex
Luminex MAGPIX
Manufaturer: Luminex Proudct Name: MAGPIX Catalog #: MAGPIX-XPONENT
Ordering Information
US Customer Ordering Information (Research Use Only)
Luminex Platform
Product Name: QuantiDNA™ Direct cfDNA Test
Pack size: 96 Reactions
Catalog Number: DC-08-0096R
The CE/IVD marked version and the Research Use Only version have the same contents & configuration.
Outside US Customer Ordering Information (CE/IVD Marked)
Luminex Platform
Product Name: QuantiDNA™ Direct cfDNA Test
Pack size: 96 Reactions
Catalog Number: DC-08-0096E
The CE/IVD marked version and the Research Use Only version have the same contents & configuration.
US Customer Ordering Information (Research Use Only)
Luminometer Platform
Product Name: QuantiDNA™ DNA Measurement Assay
Pack size: 96 Reactions
Catalog Number: DC-08-0092R
The CE/IVD marked version and the Research Use Only version have the same contents & configuration.
Outside US Customer Ordering Information (CE/IVD Marked)
Luminometer Platform
Product Name: QuantiDNA™ DNA Measurement Assay
Pack size: 96 Reactions
Catalog Number: DC-08-0092E
The CE/IVD marked version and the Research Use Only version have the same contents & configuration.
